Special Interest Group Update
In each issue, one of NANN’s special interest groups will share information in their area of focus.
Spina Bifida and Post-NICU Outcomes
Julie E. Williams, DNP CRNP NNP-BC
Spina bifida is the most common congenital anomaly of the central nervous system that is compatible with life (Ntimbani, Kelly, & Lekgwara, 2019). Spina bifida is the failure of a developing neural tube to close during the fourth week of gestation. The incomplete closure of the neural tube can occur anywhere along the spine. When this occurs, the bone that protects the spinal cord does not form, leaving the spinal cord and nerves exposed to the amniotic fluid.
Incidence
Spina bifida affects approximately 1,645 infants every year (Centers for Disease Control and Prevention [CDC], 2018). Hispanic women have the highest rate of spina bifida when compared to non-Hispanic white and non-Hispanic black women. The prevalence of spina bifida was 3.8 per 10,000 live births for Hispanic women, 2.73 per 10,000 live births for non-Hispanic African-American women, and 3.09 per 10,000 live births for non-Hispanic white women (CDC, 2018).
Classification
Spina bifida encompasses two categories: spina bifida aperta (primary neural tube defect) and spina bifida occulta (secondary neural tube defects) (Papachrisanthou, 2019; Hansen & Warf, 2017). Spina bifida aperta, or open spina bifida, represents the majority of neural tube defects. Spina bifida occulta, or closed spina bifida, is the mildest form of neural tube defect (Hansen & Warf, 2017).
Etiology and Diagnosis
The etiology of spina bifida is unknown; however, contributing factors include folic acid deficiency, anticonvulsant therapy with carbamazepine and valproic acid, antineoplastic drug therapy with aminopterin or other folic acid antagonists, antimalarial drugs, and maternal diabetes (Hansen & Warf, 2017). The defect usually occurs between the third and fourth weeks of gestation. Diagnosis can be made prenatally from elevated alpha-fetoprotein blood or amniotic fluid levels or visualization of the abnormality on ultrasound (Papachrisanthou, 2019). Postnatal diagnosis is by direct visualization of the defect or ultrasound based on clinical findings of a spinal hemangioma, hairy patch, deep dimple, or sinus tract (Hansen & Warf, 2017).
Clinical Manifestations
Clinical manifestations depend on the type of defect. Myelomeningocele (myelo = cord, meninges = covering, and cele = sac) is the most common open spinal defect (Lissauer, Fanaroff, Miall, & Fanaroff, 2016). This defect consists of a saccular outpouching containing cerebrospinal fluid (CSF), part of the spinal cord and nerves (Lissauer, Fanaroff, Miall, & Fanaroff, 2016). The infant can have moderate to severe disabilities based on the location and size of the defect. Complications include hydrocephalus from Chiari malformation (herniation of the cerebellar vermis through the foramen magnum), hip dysplasia, talipes equinovarus (clubfeet), neurogenic bladder and bowel, sensory deficits of the legs, and paralysis of the legs (Lissauer, Fanaroff, Miall, & Fanaroff, 2016).
Meningoceles present as an outpouching sac filled with CSF and dura. There is no involvement of the spinal cord or nerves. Infants with meningocele may have minor disabilities, including bone and soft tissue abnormalities (Hansen & Warf, 2017).
Spina bifida occulta can present with a skin lesion over the lower spine. Further diagnostic testing usually is warranted and includes a spinal ultrasound or MRI (Lissauer, Fanaroff, Miall, & Fanaroff, 2016). The spinal cord and nerves usually are normal (CDC, 2018). As such, there usually is no neurological deficit at birth; however, there can be tethering of the spinal cord during childhood (Lissauer, Fanaroff, Miall, & Fanaroff, 2016).
Management
In utero repair of the defect is associated with lower rates of ventriculoperitoneal shunting, a reversal of hindbrain herniation and improved nerve function and development, compared to infants who receive surgery after birth (Hansen & Warf, 2017; Johns Hopkins Medicine, 2019). For infants who undergo postnatal repair, a C-section is the preferred mode of delivery as it decreases the chances of rupturing the meningeal sac. Preoperative management includes the maintenance of prone positioning with a moist sterile dressing over the defect, head ultrasound, antibiotics to prevent infection, placement of a foley or intermittent catheterization, latex allergy precautions, and urgent closure of open defects (Hansen & Warf, 2017).
Postsurgical management and follow up are equally as important if not more important than preoperative management. The infant should remain prone or side-lying until the surgical site heals. A magnetic resonance imaging scan should be obtained, hearing and vision screens should be performed before discharge, and daily head circumference, weight, and intake/output should be followed closely. Other postoperative care should include clean intermittent catheterization (CIC), neurology and neurosurgical services, genetic services, orthopedic services, urology services, physical therapy, and social services.
Long-Term Outcome
Children with myelomeningocele require a comprehensive multidisciplinary team of providers. Services including neurosurgical, urological, orthopedic, gastroenterology and nutrition, and rehabilitation should be a part of the care. If these services can be coordinated through a spina bifida follow-up clinic or a provider versed in the care of the infant with spina bifida, there is a chance to maximize the development of the child.
Neurosurgical services should be involved as many of the children (20%–50%) with myelomeningocele will experience tethered cord syndrome where their spinal cord becomes fastened to immovable structures like overlying membranes and vertebrae (National Institute of Health, 2019). This condition causes an abnormal stretching of the spinal cord as the child grows. Clinical manifestations include back and leg pain, hypertonia, decreased sensation, a loss of muscle function to the leg, worsening scoliosis, and changes in bowel and bladder function (Phillips, Burton, & Evans, 2017). Approximately 75%–85% of infants with spina bifida and hydrocephalus require ventricular shunt placement to maintain normal intracranial pressures (Phillips, Burton, & Evans, 2017). It is not uncommon for children with hydrocephalus to require shunt revisions to remedy infections or shunt malfunction as they grow (Hansen & Warf, 2017).
Approximately 85% of the children with myelomeningocele require CIC, and 80% are able to achieve social bladder continence (Hansen & Warf, 2017). The sacral nerves (s2-s4), which control the bladder, are almost abnormal, yielding a neurogenic bladder and poor bladder emptying (Phillips, Burton, & Evans, 2017). Generally, kidney function is normal at birth; however, high bladder pressures from poor emptying can lead to vesicoureteral reflux, hydronephrosis, and consequently kidney damage. Urinary tract infections are common, and prophylactic antibiotics may be prescribed. Neurogenic bladder management inclusive of CIC and anticholinergic medications to decrease bladder spasm, periodic renal and bladder scans to monitor for hydronephrosis and bladder wall thickening, are necessary to preserve kidney function. The incorporation of urological services can facilitate this process.
Orthopedic services should be involved to manage musculoskeletal comorbidities. The higher the level of the defect on the spine, the more muscles affected, and the greater the weakness. Muscle imbalance around the joints can lead to an array of complications including hip dysplasia, talipes equinovarious, calcaneus (an imbalance between active dorsiflexion and paralyzed plantar flexion), vertical talus (rocker-bottom foot), and scoliosis among other problems (Phillips, Burton, & Evans, 2017). Children may have mobility issues from difficulty walking to paraplegia. Management of worsening scoliosis or kyphosis is critical as it can cause restrictive lung disease. Surgical intervention may be indicated to improve mobility.
The child may require the gastroenterology and nutritional services to monitor growth and nurtrition intake as these infants are at high risk for growth failure. Some infants may require a gastrostomy tube to meet their nutritional requirement and prevent aspiration (Hansen & Warf, 2017). Bowel incontinence and constipation are common problems, and an aggressive bowel program may be required.
Rehabilitation services are critical to maximizing the potential outcomes of the infant with spina bifida. Access to early intervention programs should be provided as soon as possible. These services should include care from a physical therapist, occupational therapist, and speech-language therapist. Approximately 75% of children with myelomeningocele require some variation of special education (Hansen & Warf, 2017). Learning disabilities include difficulties processing language as well as visual, perceptual, and fine motor deficits. Hydrocephalus, shunt infections, and seizures may increase the risk of cognitive delays.
Children with spina bifida can lead active lives. Prognosis is dependent on the severity of the defect and the additional services to which the child has access. In addition to medical services, the family should be referred to community resources to help them with the emotional aspects of caring for a child with spina bifida.
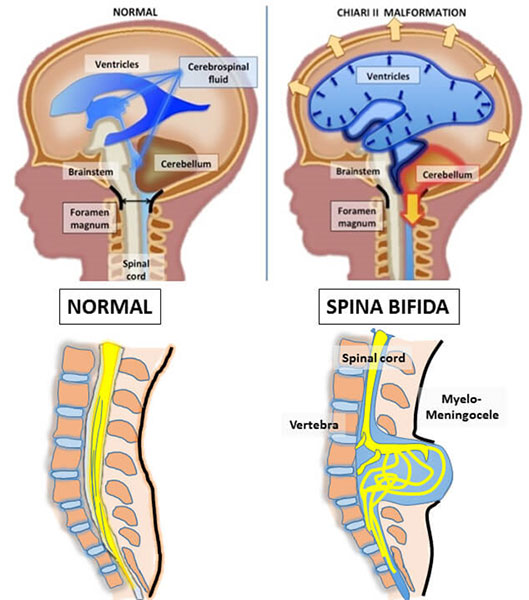
Photo retrieved from https://www.hopkinsmedicine.org/gynecology_obstetrics/specialty_areas/fetal_therapy/conditions-we-treat/spina_bifida.html
References
Hansen, A., & Warf, B. (2017). Neural tube defects. In E. Eichenwald, A. Hansen, C. Martin, & A. Stark (Eds.), Cloherty and Starks's manual of neonatal care (8th ed., pp. 829-843). Philadelphia, PA: Wolters Kluwer.
Johns Hopkins Medicine. (n.d.). Spina bifida treatment.Retrieved from https://www.hopkinsmedicine.org/gynecology_obstetrics/specialty_areas/fetal_therapy/conditions-we-treat/spina_bifida.html
Lissauer, T., Fanaroff, A. A., Miall, L., & Fanaroff, J. (2016). Neonatology at a glance (3rd ed.). West Sussex, UK: John Wiley & Sons, Ltd.
National Institute of Health. (2019). Spina bifida fact sheet. Retrieved from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Spina-Bifida-Fact-Sheet#3258_10
Ntimbani, J., Kelly, A., & Lekgwara, P. (2019). Myelomeningocele - A literature review. Interdisciplinary Neurosurgery. Retrieved from https://doi.org/10.1016/j.inat.2019.100502
Phillips, L. A., Burton, J. M., & Evans, S. H. (2017). Spina bifida management. Current Problems in Pediatric and Adolescent Health Care, 47(7), 173-177.
Papachrisanthou, M. M. (2019). What does that mean: My baby has spina bifida? International Journal of Childbirth Education, 34(2), 30. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=135888086&site=ehost-live&scope=site


